


Heat (whether it was consumed or evolved) can also be an indicator that reaction occurred, but you may not be able to tell in these videos. You will know that a reaction occur if a precipitate, a gas, or color change occurred.
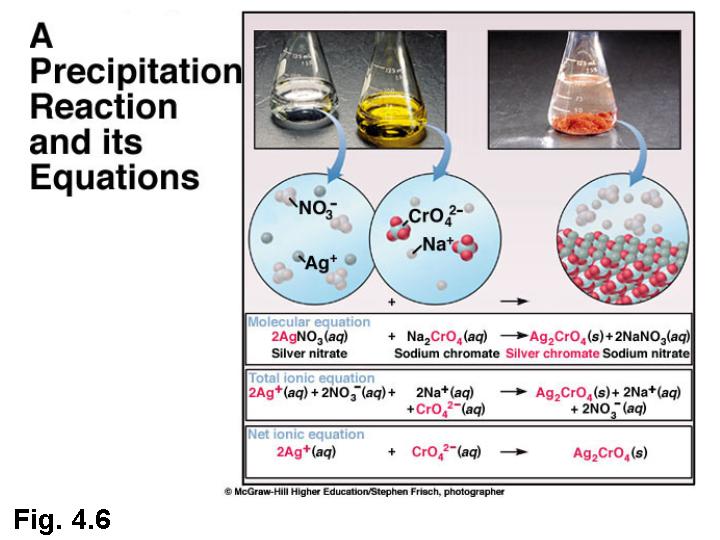
Keep in mind that some reactions will not occur and you should write NR (for No Reaction). Indicate if a gel is produced or crystals form, if the solid was powdery, etc. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Don't write “became cloudy” or “white solid”. Precipitation Reactions and Solubility Rules. Before proceeding with this or any other experiment students must sign the chemical lab safety form.įor each reaction in Part A and Part B record your observations, molecular equation, total ionic equation and net ionic equation. Make sure to write any evidence of a chemical reaction with sufficient detail to help you distinguish between similar precipitation reactions.
#Precipitate definition reactions worksheet skin#
Clean up all spills immediately! If contact with skin rinse with water for 15 minutes.Do non ingest any chemicals or inhale the vapors.Safety if you were to complete this lab in person: single replacement reactions, a type of redox reaction (text section 4. acid base neutralization reactions (text section 4.5) 3. precipitations reactions (text section 4.4) 2. Compounds that do not dissolve in water remain a solid and indicated by "(s)". Chemical Reactions in Solution Three common types of chemical reacitons that take place in solution are: 1. You might have heard that water is the universal solvent, however, water only dissolves substances that are hydrophilic (from the Greek "hydros" - water and "philia" - bonding or friendship). For example, NaCl(aq) present as individual ions Na + and Cl - dissolved in water. In chemistry aqueous solution indicated by adding "(aq)" to the reactant formula. Rain, vinegar, orange juice are all examples of aqueous solutions that you come across in your everyday life. 3.1: Introduction to Chemical EquationsĪqueous solution is any solution where water is present as a solvent.Calculate the concentration of a solution based on experimental (gravimetric) data.Design an experiment to determine the concentration of a known solution of an ionic species utilizing solubility rules and stoichiometry.Write molecular, ionic, and net ionic equations for various reactions.Many reactions of this type involve the exchange of. Predict when a chemical reaction will result in the formation of a gas. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products.Predict if a precipitate will form when combining two solutions.Record detailed observations for a reaction.Describe precipitation reactions from the molecular perspective.\)īy the end of this lab, students should be able to:


 0 kommentar(er)
0 kommentar(er)
